Clinical Data & Safety
REVIEW THE RESULTS
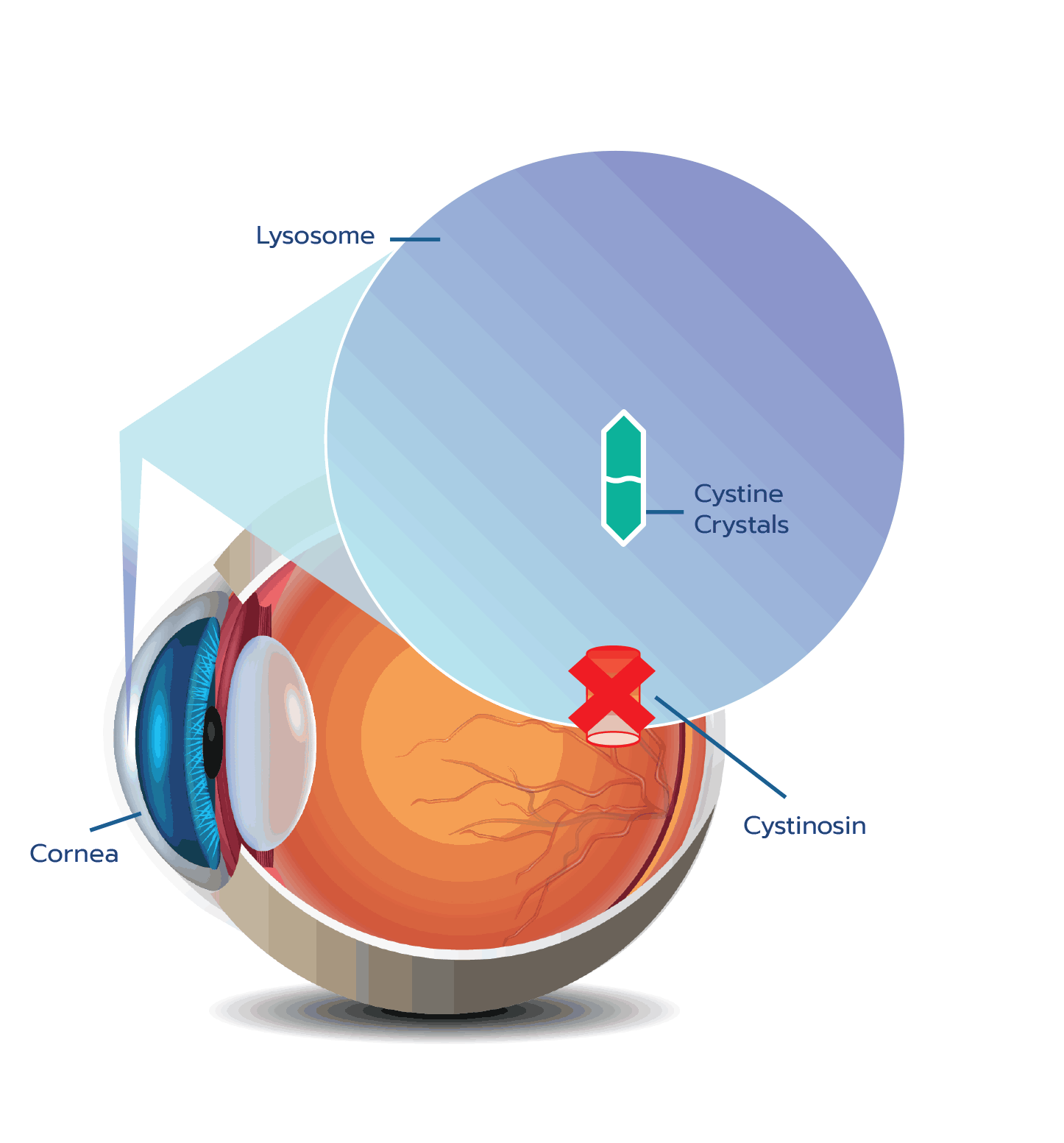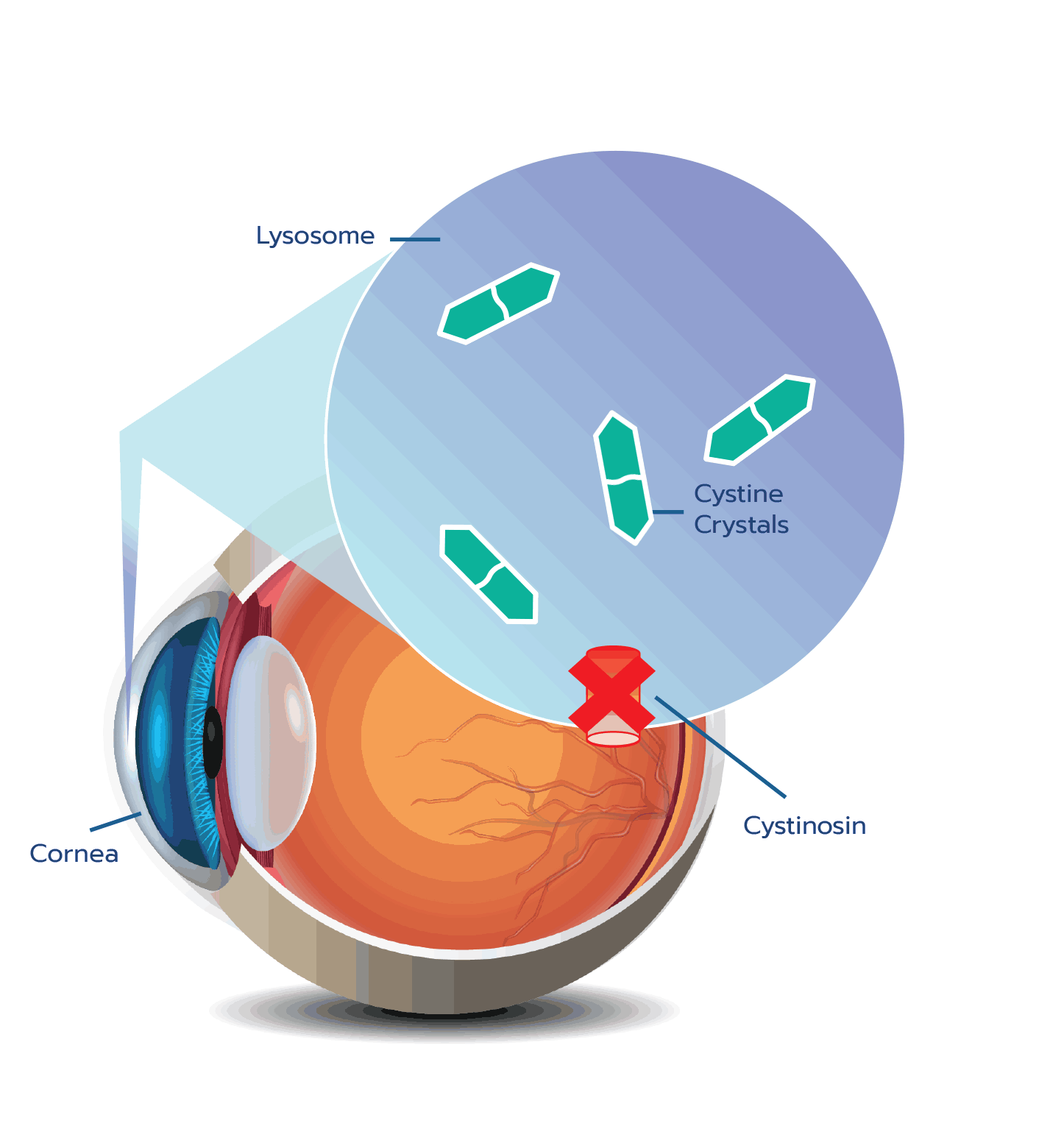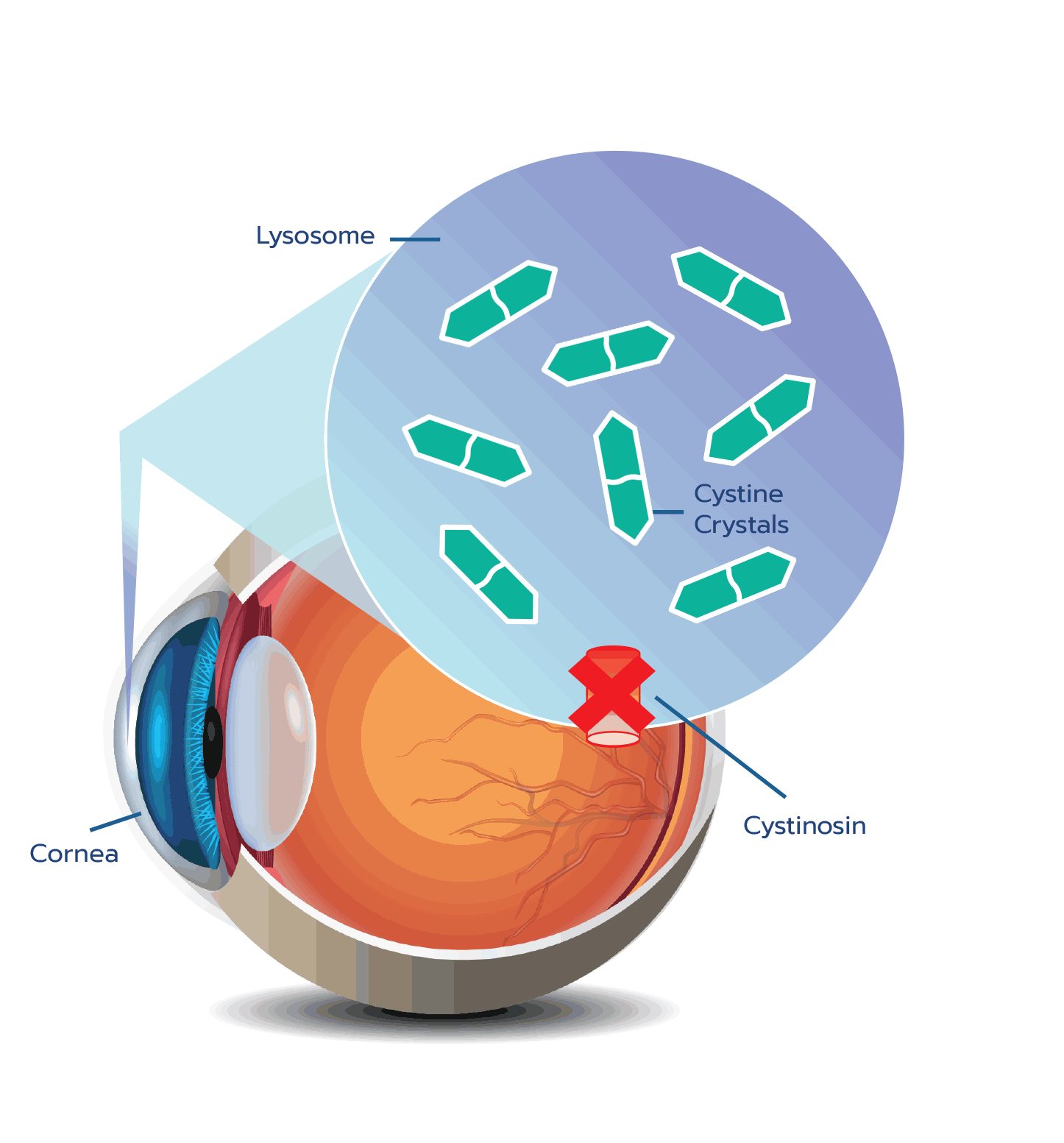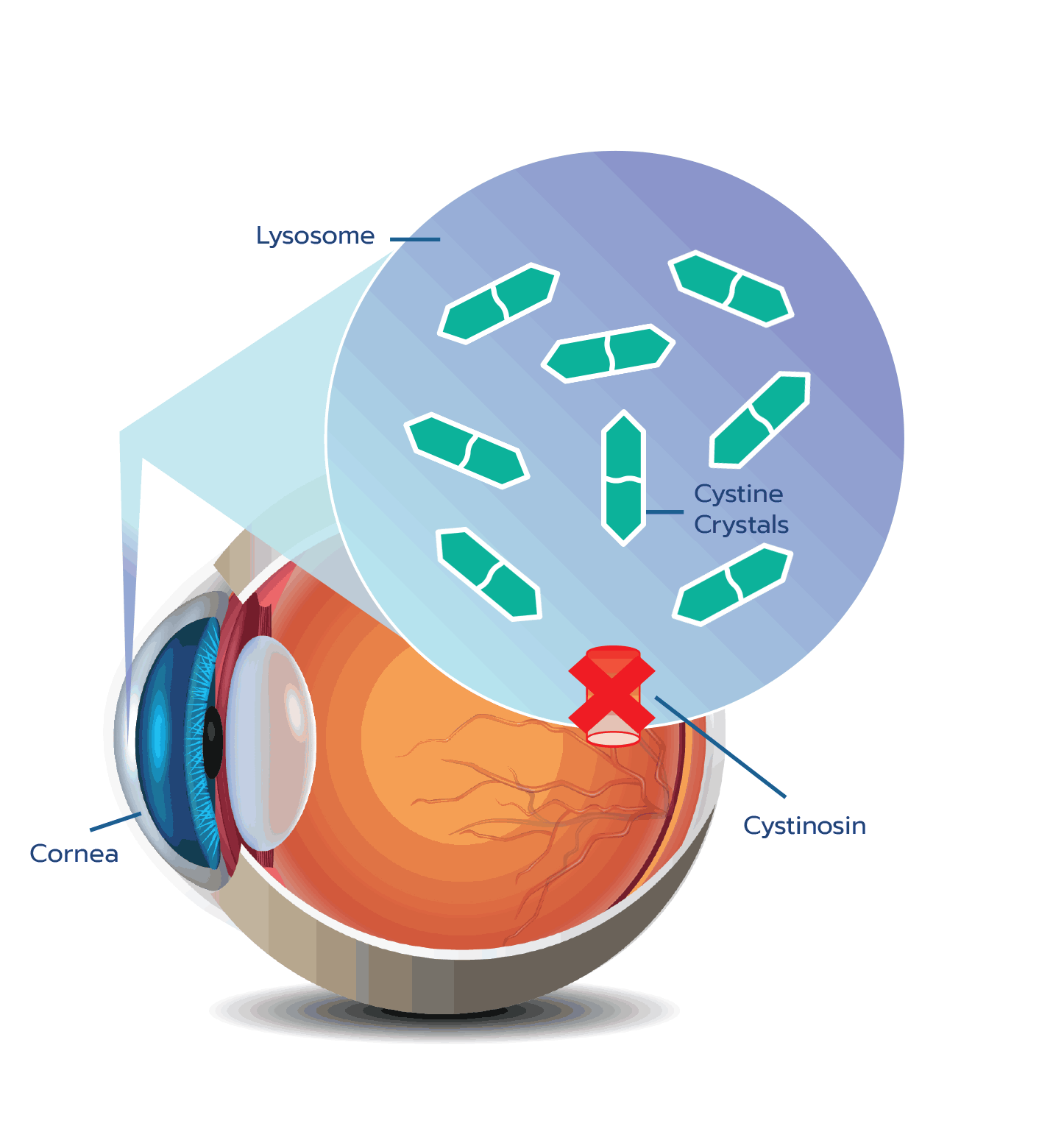
Cystinosis is an autosomal recessive lysosomal storage disease caused by mutations in the CTNS gene.1
CTNS encodes a cystine-specific transporter, cystinosin, which normally transports the amino acid cystine out of
CTNS mutations result in absent or defective cystinosin, which prevents the normal transport of cystine out of the
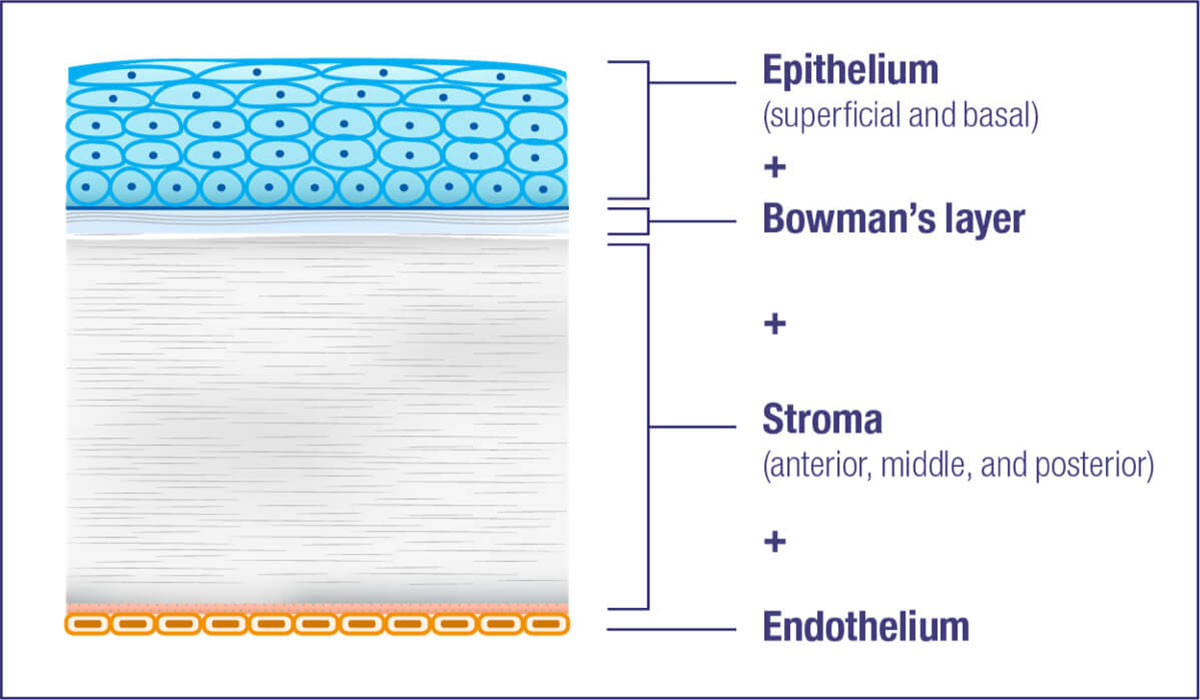
Cystine crystal formation negatively impacts many organs throughout the body and leads to significant health complications. One of the organs impacted is the eye.4,5 In the eye, cystine crystals appear most often in the
Although ocular symptoms may appear later in life than many other symptoms of cystinosis, ocular symptoms can impair quality of life in untreated patients, especially as cystine crystals accumulate over time.5,6,10
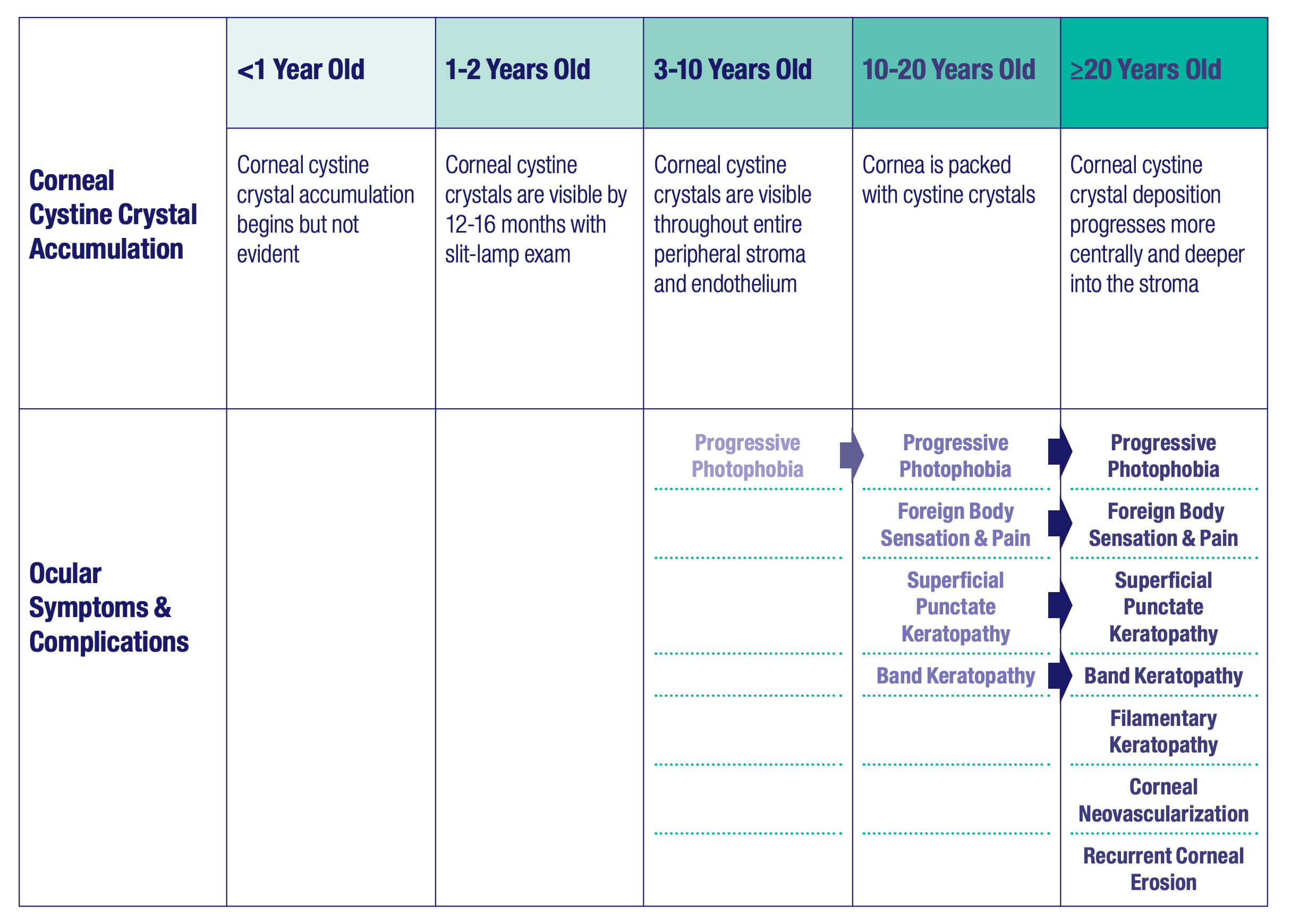
Cystine crystal deposition begins in the peripheral
*Reproduced by permission from Labbe 2014
Corneal cystine crystals are not detectable at birth, but they can generally be observed via slit-lamp examination by the age of 12-16 months.12,14 As the disease progresses, by approximately 7 years of age, the entire peripheral stroma and endothelium accumulate crystals. By approximately 20 years of age, crystals can be seen in the entire corneal stroma.14
TARGETED TREATMENT FOR CORNEAL CYSTINE CRYSTALS IS NEEDED.
Cysteamine eye drops may effectively deplete corneal cystine crystals and, as a result, decrease or lessen the risk of developing uncomfortable or debilitating symptoms.8,14,18 Due to the cornea’s avascularity, systemic cysteamine does not affect corneal cystine crystal accumulation.12,18,19
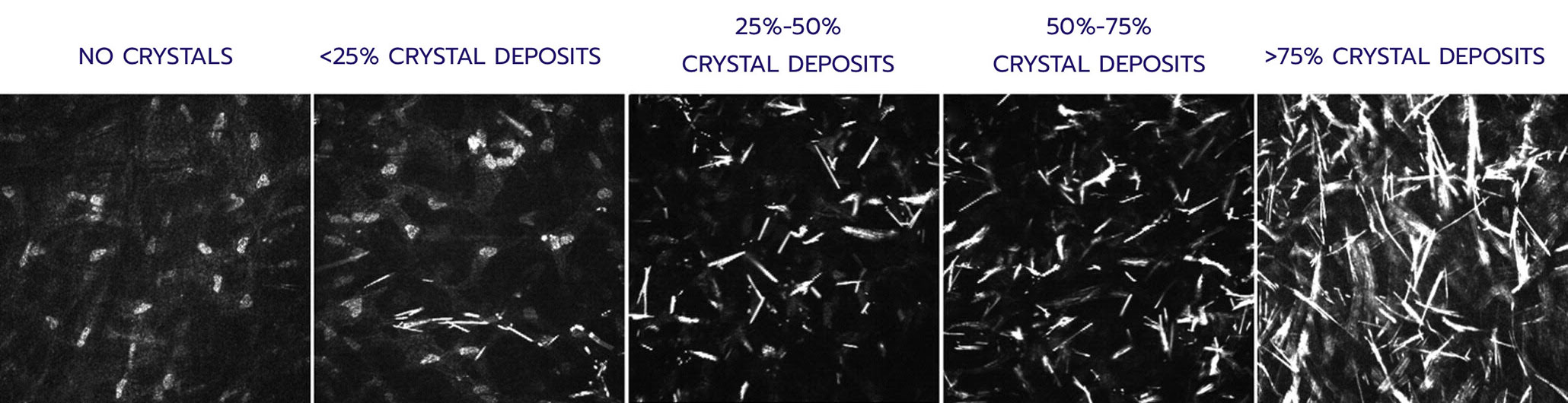
Quantifies: Corneal cystine crystal density at the cellular level in 7 layers of the
IVCM Scoring: 0-4 in each layer
Total score: Sum of each layer’s mean score (0-28)
CYSTADROPS (cysteamine ophthalmic solution) 0.37% is a cystine-depleting agent indicated for the treatment of corneal cystine crystal deposits in adults and children with cystinosis.
INDICATIONS AND IMPORTANT SAFETY INFORMATION