Contact Recordati Rare Diseases Representative for additional information and ongoing support
RESOURCES FOR YOUR PRACTICE AND YOUR PATIENTS
These downloadable resources provide important information about CYSTADROPS, getting your patient started on treatment, and the correct use of CYSTADROPS.
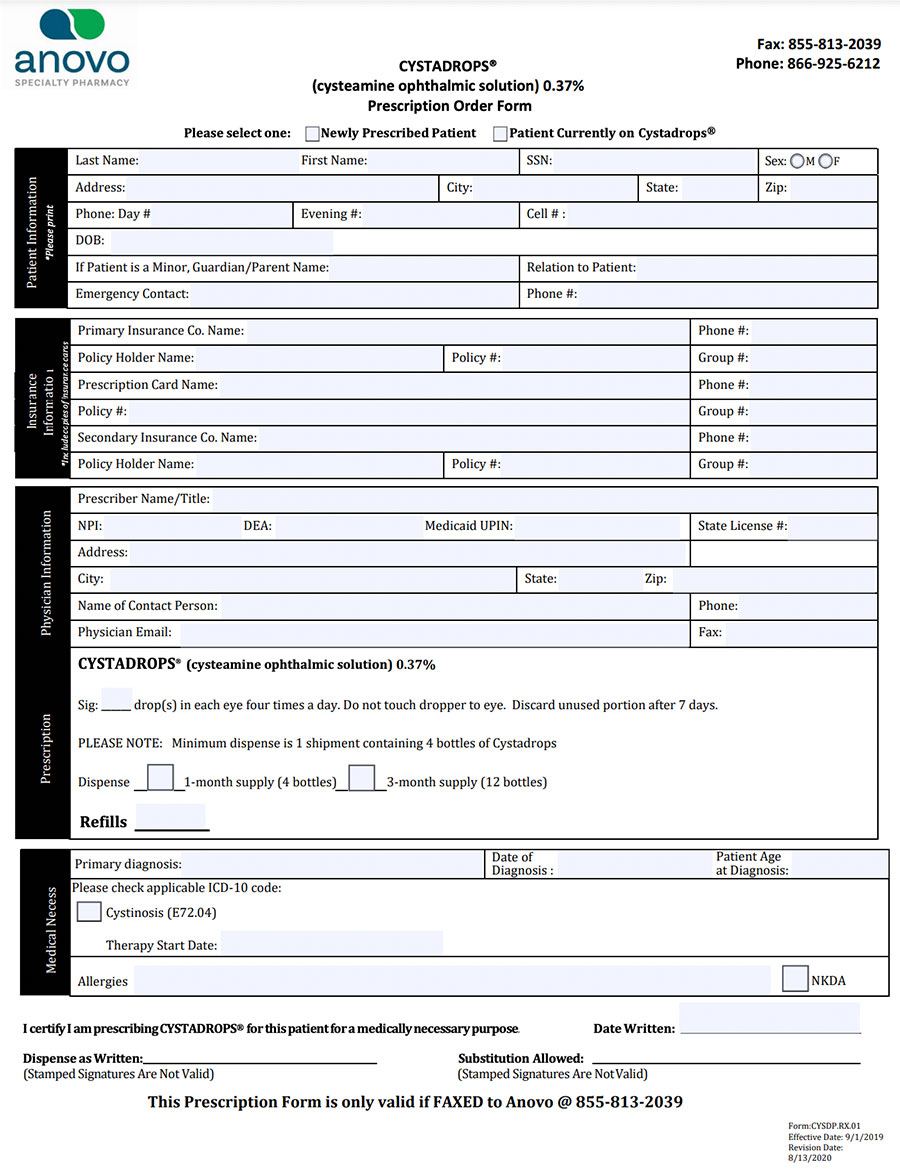
Cystadrops Treatment Form
To initiate treatment with CYSTADROPS, submit the form by fax to Anovo Specialty Pharmacy (855)-813-2039.
Starting your patients on CYSTADROPS
Helpful tips for healthcare providers initiating treatment with CYSTADROPS.
For your CYSTADROPS patients: Patient Liaison (PL) Program Opt-In Form
To enroll, your patients or their caregivers can complete the opt-in form available via the link below.
Indications and Usage
CYSTADROPS (cysteamine ophthalmic solution) 0.37% is a cystine-depleting agent indicated for the treatment of corneal cystine crystal deposits in adults and children with cystinosis.
Important Safety Information
- To minimize the risk of contamination, do not touch the dropper tip to any surface. Keep bottle tightly closed when not in use.
- Benign intracranial hypertension (or pseudotumor cerebri) associated with oral cysteamine or ophthalmic use of cysteamine (with concurrent oral cysteamine) has been reported, which has resolved with diuretic therapy.
- Contains benzalkonium chloride. Contact with soft contact lenses should be avoided. Remove contact lenses prior to application. Lenses may be reinserted 15 minutes following administration.
- The most common adverse reactions (≥ 10%) are eye pain, vision blurred, eye irritation, ocular hyperaemia, instillation site discomfort, eye pruritus, lacrimation increased, and ocular deposits.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1‑800-FDA-1088 or www.fda.gov/medwatch.
INDICATIONS AND IMPORTANT SAFETY INFORMATION
Important Safety Information
- To minimize the risk of contamination, do not touch the dropper tip to any surface. Keep bottle tightly closed when not in use.